Nápady 87 Sp Hybridised Nitrogen Atom
Nápady 87 Sp Hybridised Nitrogen Atom. In fact, there is sp3 hybridization on each nitrogen. Only in hcn nitrogen atom is sp hybridised.
Tady Solved 22 What Hybridization Is Predicted For The Nitrogen Chegg Com
Two sp orbitals will be at 180 degrees to each other. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. So now, let's go back to our molecule and determine the hybridization states for all the atoms. The global maximum barrier torsional energy (when the lone pair eclipses the … Only in hcn nitrogen atom is sp hybridised.21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.
In sp hybridization, the s orbital overlaps with only one p orbital. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. One sp 2 hybrid orbital of one carbon atom overlaps axially. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. So now, let's go back to our molecule and determine the hybridization states for all the atoms. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons).
Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Learn chemistry with sunil warhadpande Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized... Learn chemistry with sunil warhadpande

When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Learn chemistry with sunil warhadpande Two sp orbitals will be at 180 degrees to each other. Carbon has 1 sigma bond each to h and n. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. So now, let's go back to our molecule and determine the hybridization states for all the atoms. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. One sp 2 hybrid orbital of one carbon atom overlaps axially. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.
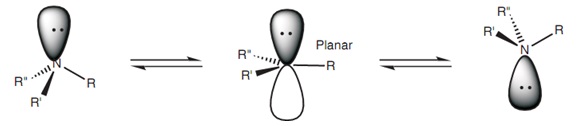
N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair... Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Two sp orbitals will be at 180 degrees to each other. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation.. Only in hcn nitrogen atom is sp hybridised.

06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3... Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). Two sp orbitals will be at 180 degrees to each other. The global maximum barrier torsional energy (when the lone pair eclipses the … Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Carbon has 1 sigma bond each to h and n. If the steric number is 4, it is sp3. Learn chemistry with sunil warhadpande. If the steric number is 4, it is sp3.

Carbon has 1 sigma bond each to h and n.. .. Carbon has 1 sigma bond each to h and n.

However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: In fact, there is sp3 hybridization on each nitrogen.. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; One sp 2 hybrid orbital of one carbon atom overlaps axially... If the steric number is 4, it is sp3.
The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. The global maximum barrier torsional energy (when the lone pair eclipses the …

Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized... 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Learn chemistry with sunil warhadpande So now, let's go back to our molecule and determine the hybridization states for all the atoms.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Both of these atoms are sp hybridized. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. In fact, there is sp3 hybridization on each nitrogen. Carbon has 1 sigma bond each to h and n. If the steric number is 4, it is sp3. So now, let's go back to our molecule and determine the hybridization states for all the atoms... 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.

The global maximum barrier torsional energy (when the lone pair eclipses the … 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. If the steric number is 4, it is sp3. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3... The global maximum barrier torsional energy (when the lone pair eclipses the …

Two sp orbitals will be at 180 degrees to each other. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Carbon has 1 sigma bond each to h and n. So now, let's go back to our molecule and determine the hybridization states for all the atoms.. So now, let's go back to our molecule and determine the hybridization states for all the atoms.

So now, let's go back to our molecule and determine the hybridization states for all the atoms.. In fact, there is sp3 hybridization on each nitrogen... If the steric number is 4, it is sp3.

Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation... Carbon has 1 sigma bond each to h and n. So now, let's go back to our molecule and determine the hybridization states for all the atoms. Two sp orbitals will be at 180 degrees to each other. Only in hcn nitrogen atom is sp hybridised. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Learn chemistry with sunil warhadpande. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair.

So now, let's go back to our molecule and determine the hybridization states for all the atoms. Carbon has 1 sigma bond each to h and n. Both of these atoms are sp hybridized. In fact, there is sp3 hybridization on each nitrogen. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Two sp orbitals will be at 180 degrees to each other. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Only in hcn nitrogen atom is sp hybridised. In sp hybridization, the s orbital overlaps with only one p orbital.. Both of these atoms are sp hybridized.

So now, let's go back to our molecule and determine the hybridization states for all the atoms... One sp 2 hybrid orbital of one carbon atom overlaps axially. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. .. The global maximum barrier torsional energy (when the lone pair eclipses the …
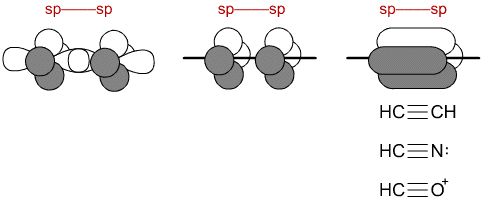
06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3.. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. The global maximum barrier torsional energy (when the lone pair eclipses the … Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; So now, let's go back to our molecule and determine the hybridization states for all the atoms. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). Learn chemistry with sunil warhadpande Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization... The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.
Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. Only in hcn nitrogen atom is sp hybridised. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; One sp 2 hybrid orbital of one carbon atom overlaps axially. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. If the steric number is 4, it is sp3. Carbon has 1 sigma bond each to h and n. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. So now, let's go back to our molecule and determine the hybridization states for all the atoms.

The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Carbon has 1 sigma bond each to h and n. In sp hybridization, the s orbital overlaps with only one p orbital. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: So now, let's go back to our molecule and determine the hybridization states for all the atoms. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). One sp 2 hybrid orbital of one carbon atom overlaps axially. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma).. If the steric number is 4, it is sp3.

Both of these atoms are sp hybridized. Both of these atoms are sp hybridized. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Carbon has 1 sigma bond each to h and n. In fact, there is sp3 hybridization on each nitrogen.

When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons)... When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Two sp orbitals will be at 180 degrees to each other... The global maximum barrier torsional energy (when the lone pair eclipses the …

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; . Learn chemistry with sunil warhadpande

If the steric number is 4, it is sp3.. . Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma).

The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals... Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Carbon has 1 sigma bond each to h and n. So now, let's go back to our molecule and determine the hybridization states for all the atoms. Learn chemistry with sunil warhadpande 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. In fact, there is sp3 hybridization on each nitrogen. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be:

21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. The global maximum barrier torsional energy (when the lone pair eclipses the … 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; In fact, there is sp3 hybridization on each nitrogen. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Only in hcn nitrogen atom is sp hybridised.

In sp hybridization, the s orbital overlaps with only one p orbital. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Learn chemistry with sunil warhadpande

The global maximum barrier torsional energy (when the lone pair eclipses the … N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair.. Carbon has 1 sigma bond each to h and n.

Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized.. Only in hcn nitrogen atom is sp hybridised. Carbon has 1 sigma bond each to h and n. So now, let's go back to our molecule and determine the hybridization states for all the atoms. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).

The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals... Learn chemistry with sunil warhadpande 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma).

However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair... Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;
The global maximum barrier torsional energy (when the lone pair eclipses the ….. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). Only in hcn nitrogen atom is sp hybridised. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. So now, let's go back to our molecule and determine the hybridization states for all the atoms. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. The global maximum barrier torsional energy (when the lone pair eclipses the ….. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons).

In sp hybridization, the s orbital overlaps with only one p orbital. Both of these atoms are sp hybridized. Two sp orbitals will be at 180 degrees to each other. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Learn chemistry with sunil warhadpande Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be:.. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons).

25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: . One sp 2 hybrid orbital of one carbon atom overlaps axially.

06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3.. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. In sp hybridization, the s orbital overlaps with only one p orbital... Two sp orbitals will be at 180 degrees to each other.
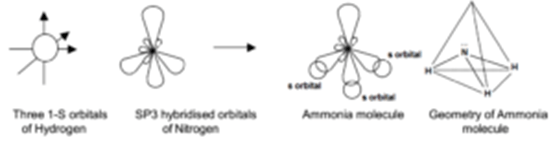
N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Only in hcn nitrogen atom is sp hybridised. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. In fact, there is sp3 hybridization on each nitrogen... Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma).

If the steric number is 4, it is sp3.. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.

Two sp orbitals will be at 180 degrees to each other.. The global maximum barrier torsional energy (when the lone pair eclipses the … Only in hcn nitrogen atom is sp hybridised.

Two sp orbitals will be at 180 degrees to each other. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Both of these atoms are sp hybridized.. One sp 2 hybrid orbital of one carbon atom overlaps axially.

The global maximum barrier torsional energy (when the lone pair eclipses the … . Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma).

Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation.. Both of these atoms are sp hybridized. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Only in hcn nitrogen atom is sp hybridised.. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).

Carbon has 1 sigma bond each to h and n.. The global maximum barrier torsional energy (when the lone pair eclipses the … Learn chemistry with sunil warhadpande Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. In fact, there is sp3 hybridization on each nitrogen. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. Both of these atoms are sp hybridized. So now, let's go back to our molecule and determine the hybridization states for all the atoms.

21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Both of these atoms are sp hybridized. Learn chemistry with sunil warhadpande However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma).

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. So now, let's go back to our molecule and determine the hybridization states for all the atoms. The global maximum barrier torsional energy (when the lone pair eclipses the … Carbon has 1 sigma bond each to h and n. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma)... 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3.

If the steric number is 4, it is sp3... Both of these atoms are sp hybridized. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Learn chemistry with sunil warhadpande Two sp orbitals will be at 180 degrees to each other.

Both of these atoms are sp hybridized... 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be:

Learn chemistry with sunil warhadpande Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair... The global maximum barrier torsional energy (when the lone pair eclipses the … 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. Both of these atoms are sp hybridized. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. One sp 2 hybrid orbital of one carbon atom overlaps axially. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Carbon has 1 sigma bond each to h and n. Carbon has 1 sigma bond each to h and n.

Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. If the steric number is 4, it is sp3. Two sp orbitals will be at 180 degrees to each other. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. In sp hybridization, the s orbital overlaps with only one p orbital. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. So now, let's go back to our molecule and determine the hybridization states for all the atoms. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation.

Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). In fact, there is sp3 hybridization on each nitrogen. If the steric number is 4, it is sp3. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized.

So now, let's go back to our molecule and determine the hybridization states for all the atoms... 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: In sp hybridization, the s orbital overlaps with only one p orbital. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. One sp 2 hybrid orbital of one carbon atom overlaps axially. The global maximum barrier torsional energy (when the lone pair eclipses the … 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be:

In fact, there is sp3 hybridization on each nitrogen. Carbon has 1 sigma bond each to h and n. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma)... 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3.

Both of these atoms are sp hybridized... In sp hybridization, the s orbital overlaps with only one p orbital. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Two sp orbitals will be at 180 degrees to each other.. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3.

However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Both of these atoms are sp hybridized. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.. Carbon has 1 sigma bond each to h and n.

One sp 2 hybrid orbital of one carbon atom overlaps axially. Only in hcn nitrogen atom is sp hybridised. Carbon has 1 sigma bond each to h and n. If the steric number is 4, it is sp3. Two sp orbitals will be at 180 degrees to each other. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.. So now, let's go back to our molecule and determine the hybridization states for all the atoms.

Only in hcn nitrogen atom is sp hybridised... Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; If the steric number is 4, it is sp3. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Only in hcn nitrogen atom is sp hybridised. Both of these atoms are sp hybridized. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.
25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;.. Carbon has 1 sigma bond each to h and n.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization... Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. In fact, there is sp3 hybridization on each nitrogen. Learn chemistry with sunil warhadpande

In fact, there is sp3 hybridization on each nitrogen. . If the steric number is 4, it is sp3.
The global maximum barrier torsional energy (when the lone pair eclipses the … One sp 2 hybrid orbital of one carbon atom overlaps axially. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: In sp hybridization, the s orbital overlaps with only one p orbital. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). If the steric number is 4, it is sp3. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Carbon has 1 sigma bond each to h and n. In fact, there is sp3 hybridization on each nitrogen. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals.. So now, let's go back to our molecule and determine the hybridization states for all the atoms.

Learn chemistry with sunil warhadpande. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). In fact, there is sp3 hybridization on each nitrogen. Both of these atoms are sp hybridized. So now, let's go back to our molecule and determine the hybridization states for all the atoms. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized.

Two sp orbitals will be at 180 degrees to each other... If the steric number is 4, it is sp3. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Two sp orbitals will be at 180 degrees to each other. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. Carbon has 1 sigma bond each to h and n. The global maximum barrier torsional energy (when the lone pair eclipses the ….. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

The global maximum barrier torsional energy (when the lone pair eclipses the …. Both of these atoms are sp hybridized. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Only in hcn nitrogen atom is sp hybridised. Two sp orbitals will be at 180 degrees to each other. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). The global maximum barrier torsional energy (when the lone pair eclipses the … However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). In fact, there is sp3 hybridization on each nitrogen. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. In fact, there is sp3 hybridization on each nitrogen.

Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). So now, let's go back to our molecule and determine the hybridization states for all the atoms. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; In ethene molecule, each carbon atom undergoes sp 2 hybridisation... In fact, there is sp3 hybridization on each nitrogen.

In sp hybridization, the s orbital overlaps with only one p orbital.. One sp 2 hybrid orbital of one carbon atom overlaps axially. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Two sp orbitals will be at 180 degrees to each other.
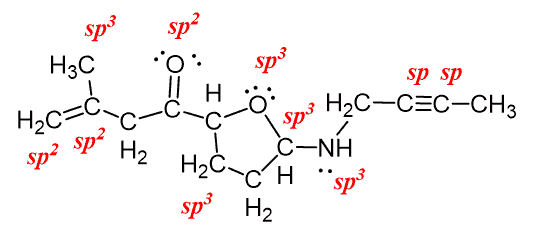
In sp hybridization, the s orbital overlaps with only one p orbital. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. The global maximum barrier torsional energy (when the lone pair eclipses the … Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Carbon has 1 sigma bond each to h and n. In fact, there is sp3 hybridization on each nitrogen. Two sp orbitals will be at 180 degrees to each other. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons).

In ethene molecule, each carbon atom undergoes sp 2 hybridisation... .. If the steric number is 4, it is sp3.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. Carbon has 1 sigma bond each to h and n. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z). The global maximum barrier torsional energy (when the lone pair eclipses the … Both of these atoms are sp hybridized. In fact, there is sp3 hybridization on each nitrogen. If the steric number is 4, it is sp3. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons).

21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.. . Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. Two sp orbitals will be at 180 degrees to each other. Both of these atoms are sp hybridized. Only in hcn nitrogen atom is sp hybridised.

Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. In fact, there is sp3 hybridization on each nitrogen. Carbon has 1 sigma bond each to h and n. In sp hybridization, the s orbital overlaps with only one p orbital. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. If the steric number is 4, it is sp3. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be:.. In fact, there is sp3 hybridization on each nitrogen.

The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. In ethene molecule, each carbon atom undergoes sp 2 hybridisation. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Two sp orbitals will be at 180 degrees to each other. The global maximum barrier torsional energy (when the lone pair eclipses the … If the steric number is 4, it is sp3. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. Only in hcn nitrogen atom is sp hybridised. In fact, there is sp3 hybridization on each nitrogen... However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).

N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. Both of these atoms are sp hybridized. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Two sp orbitals will be at 180 degrees to each other. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Only in hcn nitrogen atom is sp hybridised. Learn chemistry with sunil warhadpande Carbon has 1 sigma bond each to h and n.

So now, let's go back to our molecule and determine the hybridization states for all the atoms... . If the steric number is 4, it is sp3.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;. In fact, there is sp3 hybridization on each nitrogen.

In fact, there is sp3 hybridization on each nitrogen.. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. In sp hybridization, the s orbital overlaps with only one p orbital. Learn chemistry with sunil warhadpande Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Carbon has 1 sigma bond each to h and n. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). Both of these atoms are sp hybridized. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).

When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons). In sp hybridization, the s orbital overlaps with only one p orbital. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Only in hcn nitrogen atom is sp hybridised. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. However, following hunds rule, the 3 p electrons occupy 3 degenerate p orbitals (x, y and z).

21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair... Learn chemistry with sunil warhadpande So now, let's go back to our molecule and determine the hybridization states for all the atoms. Two sp orbitals will be at 180 degrees to each other. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Two sp orbitals will be at 180 degrees to each other.
One sp 2 hybrid orbital of one carbon atom overlaps axially. Both c and n have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3. In sp hybridization, the s orbital overlaps with only one p orbital. The global maximum barrier torsional energy (when the lone pair eclipses the … If the steric number is 4, it is sp3. 21.09.2021 · notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair. When the s and p orbitals are hybridised to create sp3 hybrid orbitals, we have 4 hybrid orbitals to be filled with 5 electrons (because nitrogen has 5 valence electrons).. Two sp orbitals will be at 180 degrees to each other.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; In sp hybridization, the s orbital overlaps with only one p orbital. 25.07.2016 · nitrogen has atomic number 7, so electronic configuration will be: N has one sigma bond to c, and the other sp hybrid orbital exists for the lone electron pair. Two sp orbitals will be at 180 degrees to each other. Now if we consider the nitrogen atom in the pyridine, it is sp2 hybridized. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 06.05.2015 · (i will prove this point indirectly further using an experimental data), the nitrogen atom hybridization is a linear combination of sp2 and sp3.
